Living Neural Network Dynamics
• Coupled Biomechanical and Ionic Excitability in Developing Neural Cell Networks
Sylvester Gates, PhD Student, Biology
with Prof. Kan Cao (Brain & Behavior Institute, UMD), and Dr. Kate O'Neill, Research Scientist
Waves and oscillations play a key role in the flow and processing of information in the brain. Recent work has demonstrated that in addition to electrical activity,
biomechanical signaling can also be excitable and thus capable of self-sustaining oscillations and waves. Here we measured the biomechanical dynamics of actin
polymerization in neural precursor cells throughout their differentiation into populations of neurons and astrocytes. Fluorescence-based live-cell imaging allowed
us to analyze the dynamics of actin in conjunction with the dynamics of calcium signals. Actin dynamics throughout differentiation showed a rhythmic character,
localized mostly in processes, with changes in scale associated with differentiation. Furthermore, actin dynamics impact ionic dynamics, with an increase in the
frequency of calcium bursts accompanied by a decrease in cell-cell correlations when actin dynamics is inhibited. This impact of cytoskeletal dynamics on cell-cell
coupling and ionic neural cell signaling suggests that information flow in the brain may be able to harness both biomechanical and electrical/ionic excitability.
Publications:
Gates S, Alvarez P, Cao K, O’Neill KM, Losert W, bioRxiv 2023
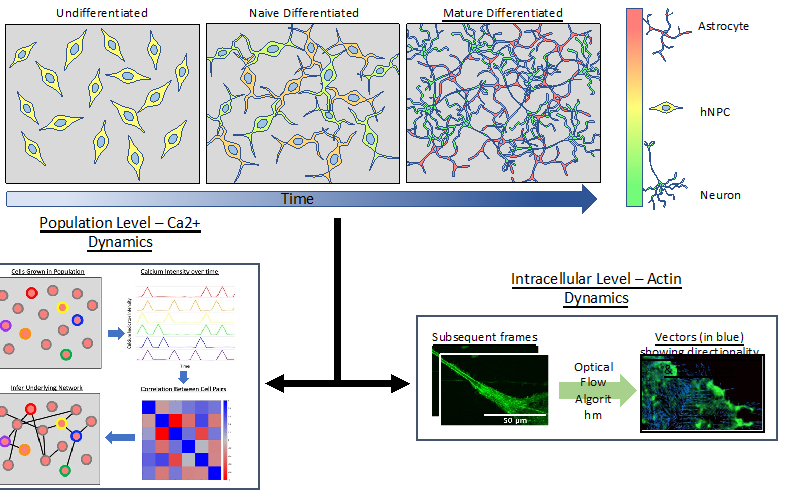
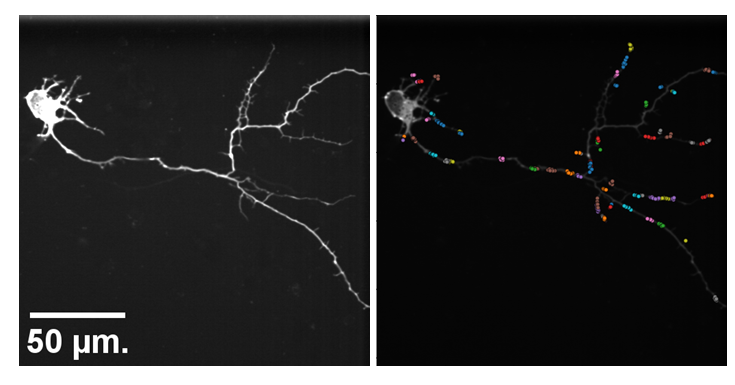
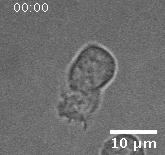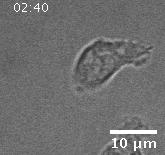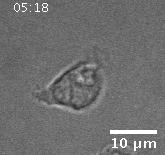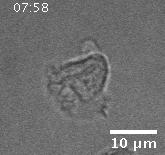
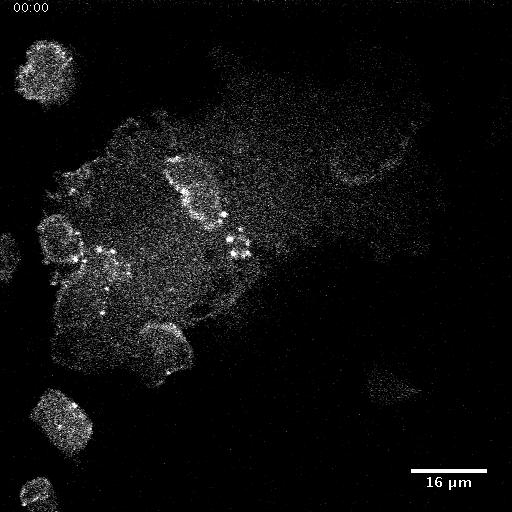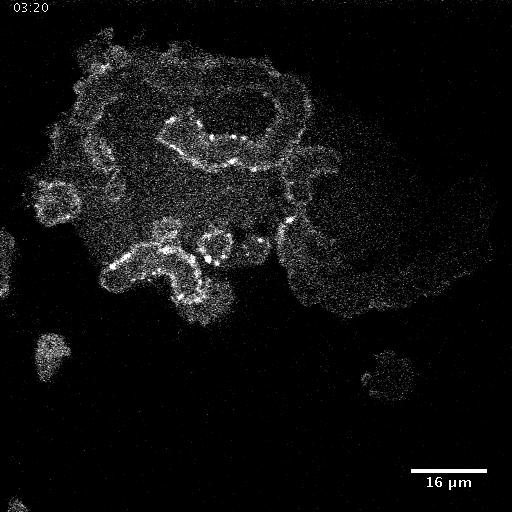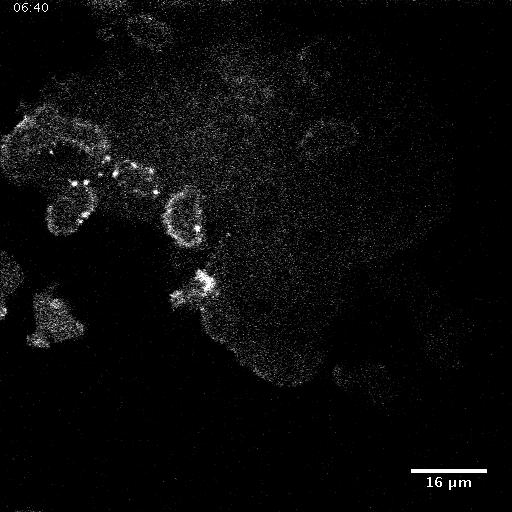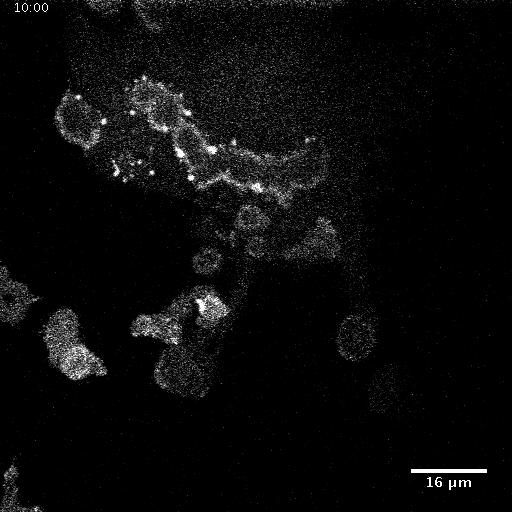
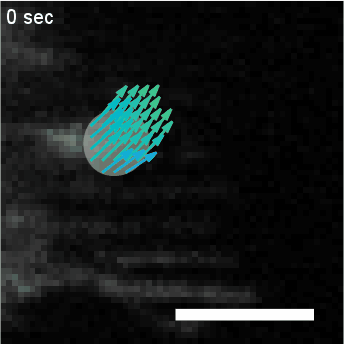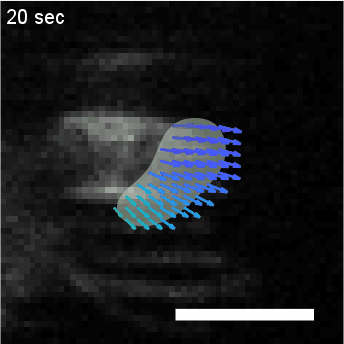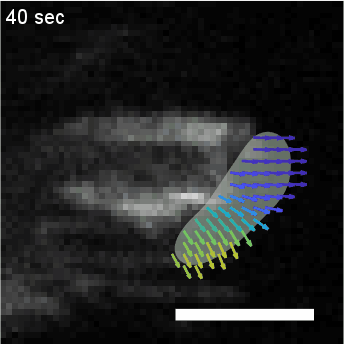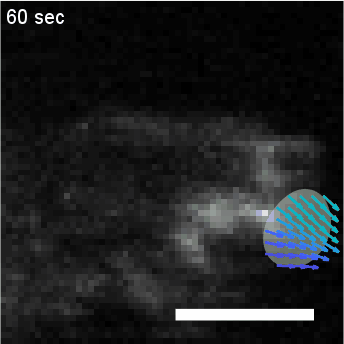
Model hNPC Line Workflow and Differentiation Capability: Starting with timecourse of hNPC differentiating towards
matured neurons and astrocytes followed by analysis of calcium and actin dynamics.
• Collective Information and Communication in Morphologically Distinct Astrocytes
Dr. Nick Mennona, Physics
with Dr. Valentina Benfenati (National Research Council of Italy), Dr. Grazia Paola Nicchia (University of Bari Aldo Moro), and Dr. Kate O'Neill, Research Scientist
Collective activity patterns in astrocytic networks are of considerable importance, given the recent finding that astrocytes actively contribute to cognition.
This work analyzes collective information capacity and information flow of calcium signals within morphologically distinct in vitro astrocyte networks, building on prior
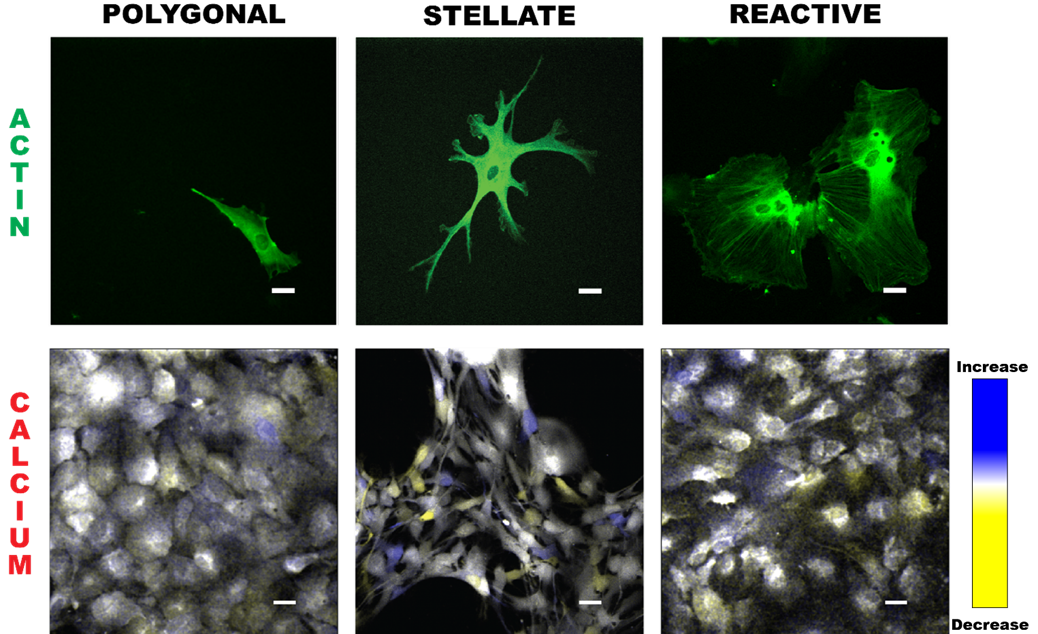
high-resolution studies of Ca2+ dynamics within individual astrocytes. We investigate group Ca2+ dynamics in several astrocytic phenotypes – polygonal, stellate, and reactive –
which represent distinct cytoskeletal architectures with differences in cell-cell coupling that may affect information flow within astrocyte networks. All morphologies are present in
the brain to varying degrees at different physiological states.
Publications:
Mennona N, Barile B, Kang H, et al., bioRxiv 2024
Astrocyte morphologies (polygonal, stellate, and reactive) are visualized through actin images (green). Time overlays illustrate calcium propagation,
with blue indicating an increase in Ca2+, yellow a decrease, and white representing values similar to the previous timepoint.
• Large Scale Collective Neural Network Dynamics
Anna Emenheiser, PhD Student, Biophysics
with Prof. Kan Cao (Brain & Behavior Institute, UMD)
We record the activity of a self-assembled network of thousands of neural cells in one field of view via calcium imaging. Since synapses between neurons and other cell-cell junctions are also mechanical in nature, we also image dynamic changes in one major mechanical element of the cell scaffolding, the actin cytoskeleton using a novel multiscale microscope system. With this biological model and multiscale imaging, we then investigate how the collective calcium and biomechanical activity changes in neural models for neurodegenerative diseases such as Alzheimer’s Disease.
Calcium dynamics in genetically modified cortical neurons. Calcium dynamics are a proxy for the electrical activity in neurons.
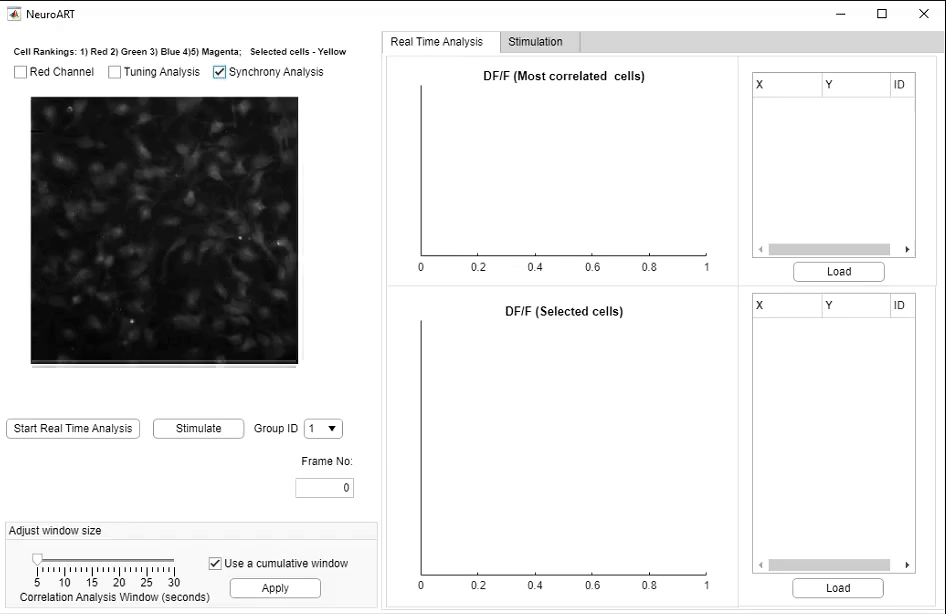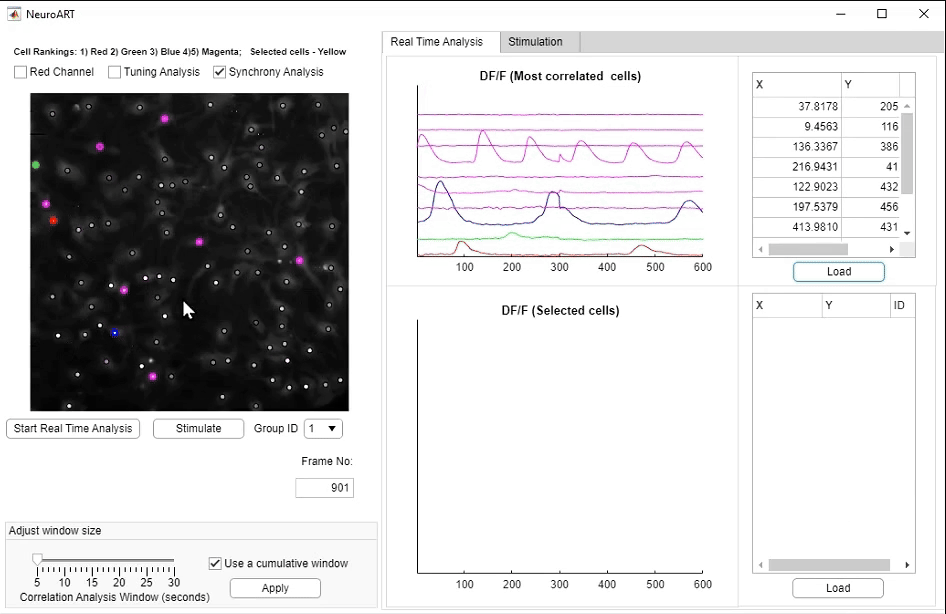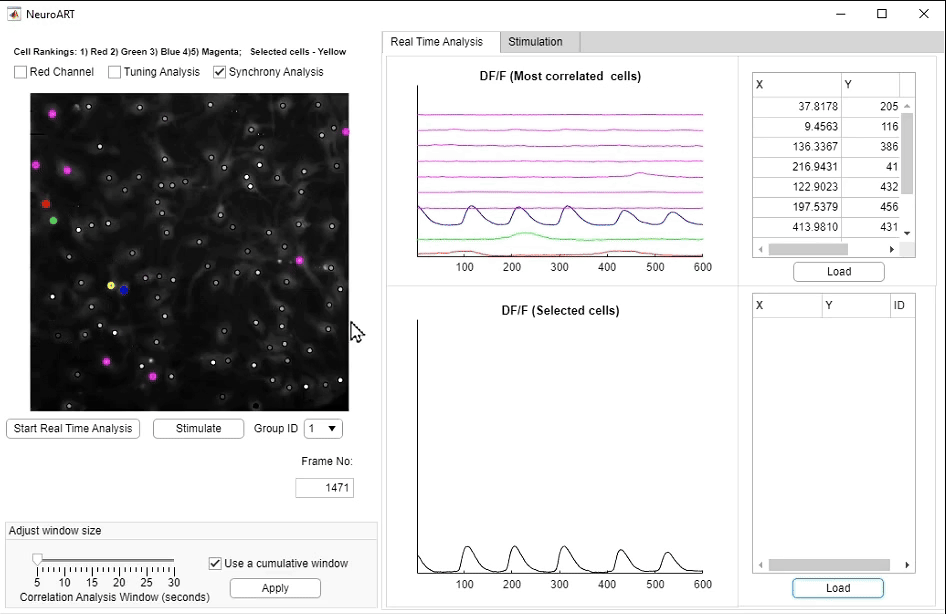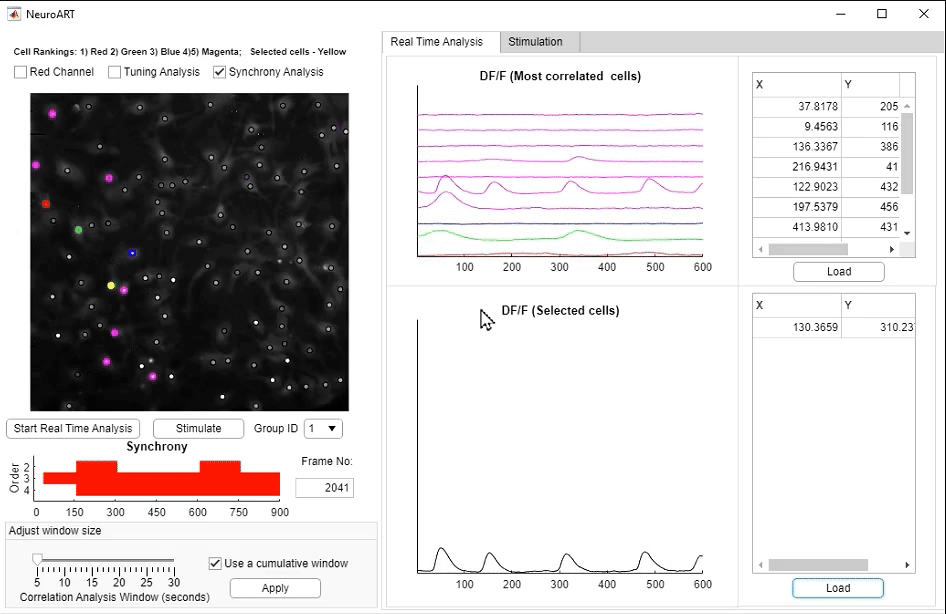
• NeuroART: Real-Time Analysis and Modulation of Neuronal Networks
Dulara De Zoysa, PhD Student, Bioengineering
with Prof. Patrick Kanold (Johns Hopkins University)
We have developed NeuroART (Neuronal Analysis in Real Time), software that provides real-time readout of neuronal activity integrated with downstream analysis of correlations and synchrony and of sensory metadata. On the example of auditory stimuli, we demonstrate real-time inference of the contribution of each neuron in the field of view to sensory information processing. To avoid the limitations of microscope hardware and enable collaboration of multiple research groups, NeuroART taps into microscope data streams without the need for modification of microscope control software and is compatible with a wide range of microscope platforms. NeuroART also integrates the capability to drive a spatial light modulator (SLM) for holographic photostimulation of optimal stimulation targets, enabling real-time modification of functional networks.
Publications:
Bowen Z, Zoysa D, et al., eNeuro 2024
NeuroART GUI for real-time analysis. Key features highlighted include the augmented image with overlaid neuronal activity, automatically identified
and manually selected neurons to closely monitor ΔF/F traces, and the photostimulation trigger for optogenetic stimulation.
• Astrocytes use dynamic actin waves and reoriented actin filaments to respond to chemophysical cues
Dr. Kate O'Neill, Research Scientist and Dr. Nick Mennona, Physics
with Dr. Valentina Benfenati (National Research Council of Italy)
with Dr. Grazia Paola Nicchia (University of Bari Aldo Moro)
Astrocytes are the most abundant glial cell and are considered non-excitable brain cells
because they do not fire action potentials like neurons. Instead, astrocytes play key roles in
regulating various aspects of brain homeostasis, which is essential for proper cognitive function.
Our work explores a less traditional aspect of excitability in the form of intracellular actin waves,
and we reveal that the homeostatic regulation of the brain by astrocytes may be more active
than previously thought. In collaboration with the National Research Council of Italy and the
University of Bari Aldo Moro, we use live imaging and novel dynamics analyses to show that
astrocytes use actin dynamics near the boundary to sense chemical and mechanical cues.
Superresolution imaging also reveals that orientation of actin filaments near the boundary may
contribute to the unique dynamics observed after specific mechanical cues.
Using superresolution imaging coupled with LoG (Laplacian of Gaussian), we demonstrate that
astrocytic actin organizes differently near the boundary depending upon the differentiated state
of the astrocyte. Astrocytes grown on HTlc (a nanotopography which induces a stellate
morphology) have actin with clusters perpendicular relative to cell boundary; astrocytes grown
on PDL have parallel actin clusters relative to their closest boundaries. These results
demonstrates that organization of actin can predict functional states in astrocytes.
Publications:
O’Neill KM, Saracino E, Barile B, Mennona NJ, et al., Advanced Biology
2022
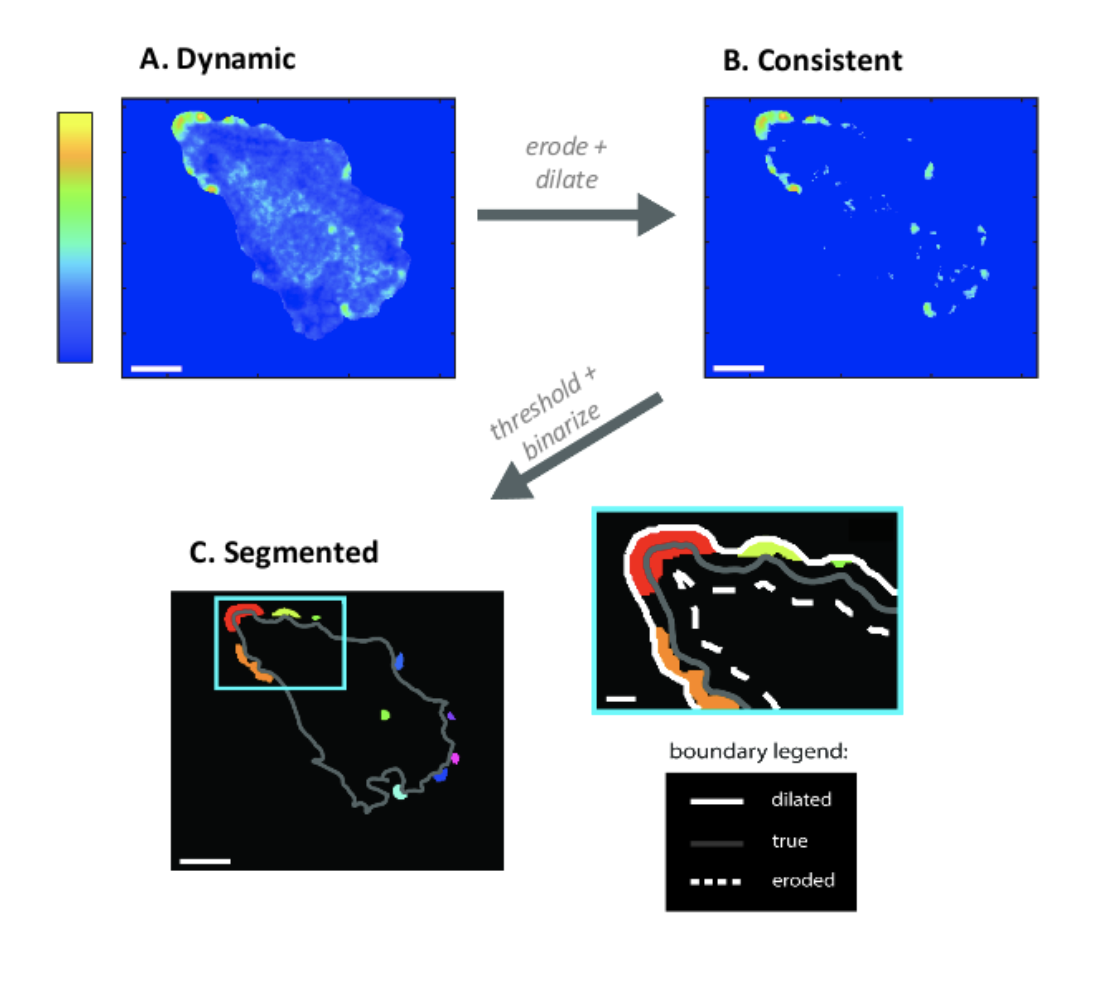
(A) Astrocytes display actin dynamics near the
boundary, both at rest and in response to
chemophysical cues. Colorbar indicates
probability of observing dynamics. (B) Dynamics
cluster into consistently active “hotspot”
regions, which tend to be near the boundary.
(C) Further processing allows use to segment
these regions for further analysis.
• Nanotopography Modulates Actin Waves and Growth Cone Navigation in Neurons
Spandan Pathak, PhD Student, Biophysics
with Dr. Kate O'Neill, Research Scientist
Axonal development relies on cytoskeletal dynamics and environmental cues, with actin remodeling driving growth cone navigation. While growth cone actin dynamics are well-studied,
their role in non-growth cone neurites is less understood. This study examines how nanotopography influences actin organization and behavior across neuronal development. We used optical flow clustering to track actin dynamics in
cortical rat neurons grown on flat versus nanoridged surfaces at various DIV stages, comparing the effects of different surface topographies and developmental time points.
Actin tracks on early developmental stage primary cortical neurons grown on flat surface.
• Actin Dynamics as a Multiscale Integrator of Cellular Guidance Cues
Dr. Abby Bull, Physics
with Prof. Min Zhao (UC Davis) and Prof. Quan Qing (ASU)
Migrating cells must integrate multiple, competing external guidance cues. However, it is not well understood how cells prioritize among these cues. We investigate external cue integration by monitoring the response of wave-like, actin-polymerization dynamics, the driver of cell motility, to combinations of nanotopographies and electric fields in neutrophil-like cells. The electric fields provide a global guidance cue, and approximate conditions at wound sites in vivo. The nanotopographies have dimensions similar to those of collagen fibers, and act as a local esotactic guidance cue. We find that cells prioritize guidance cues, with electric fields dominating long-term motility by introducing a unidirectional bias in the locations at which actin waves nucleate. That bias competes successfully with the wave guidance provided by the bidirectional nanotopographies.
Publications:
Bull A, Campanello L, Hourwitz M, et al.,
Frontiers in Cell and Developmental Biology 2022
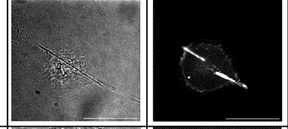
Migration of a neutrophil-like HL60 cell.
• Nanotopography modulates intracellular excitable systems through cytoskeleton actuation
Dr. Qixin Yang, Physics
with Prof. Peter Devreotes (Johns Hopkins University), and Prof. John Fourkas (University of Maryland)
Cellular sensing of most environmental cues involves receptors that affect a signal-transduction excitable network (STEN), which is coupled to a cytoskeletal excitable network (CEN).
We show that the mechanism of sensing of nanoridges is fundamentally different. CEN activity occurs preferentially on nanoridges, whereas STEN activity is constrained between nanoridges.
In the absence of STEN, waves disappear, but long-lasting F-actin puncta persist along the ridges. When CEN is suppressed, wave propagation is no longer constrained by nanoridges. A
computational model reproduces these experimental observations. Our findings indicate that nanotopography is sensed directly by CEN, whereas STEN is only indirectly affected due to a CEN-STEN
feedback loop. These results explain why texture sensing is robust and acts cooperatively with multiple other guidance cues in complex, in vivo microenvironments.
Publications:
Yang Q, Miao Y, Banerjee P, et al.,
PNAS 2023
Giant Dicty cells on a flat surface.
• Excitable Systems perspective on the cellular impact of Elongate Mineral Particles (Asbestos)
Cathy Gu, PhD Student, Physics. Jeneh Perry, PhD Student, Biology.
with Prof. Ann G. Wylie (University of Maryland), and Prof. John Fourkas (University of Maryland)
We investigate how the geometry of elongate mineral particles (EMPs) may be sensed by cells based on “esotaxis”, a recently discovered mechanism of texture sensing. Esotaxis is based on
cytoskeletal waves and oscillations that are nucleated, shaped, and steered by the texture of the surrounding. We find that EMPs trigger an esotactic response in macrophages, and that the
EMP response dominates cytoskeletal activity in these immune cells, while epithelial cells show little to no esotactic response. This result is consistent with the distinct interaction of
both cell types with ridge topographies of dimensions comparable to asbestiform EMPs. Our findings raise the question of whether narrow EMPs may also dominate cytoskeletal activity in other
immune cell types that exhibit similar esotaxis effects. These findings together with prior studies of esotaxis lead us to the hypothesis that asbestiform EMPs suppress the migration of
immune cells and activate immune signaling, lowering the ability of the immune system from reaching nearby tissue.
Publications:
Gu S, Bull A, Perry J, et al., Environmental Research 2023
(Left) Brightfield image with macrophages and asbestiform (Tremolite). (Right) Fluorescence image of polymerized actin.
• Nanotopography and Cell Clustering in Co-cultures
Cathy Gu, PhD Student, Physics. Sam Rosemore, Physiology and Neurobiology undergrad. Kashmyr Dalang, Research Assistant. Lin Zhao, PhD Student, Biophysics.
with Prof. John Fourkas (Department of Chemistry and Biochemistry, UMD)
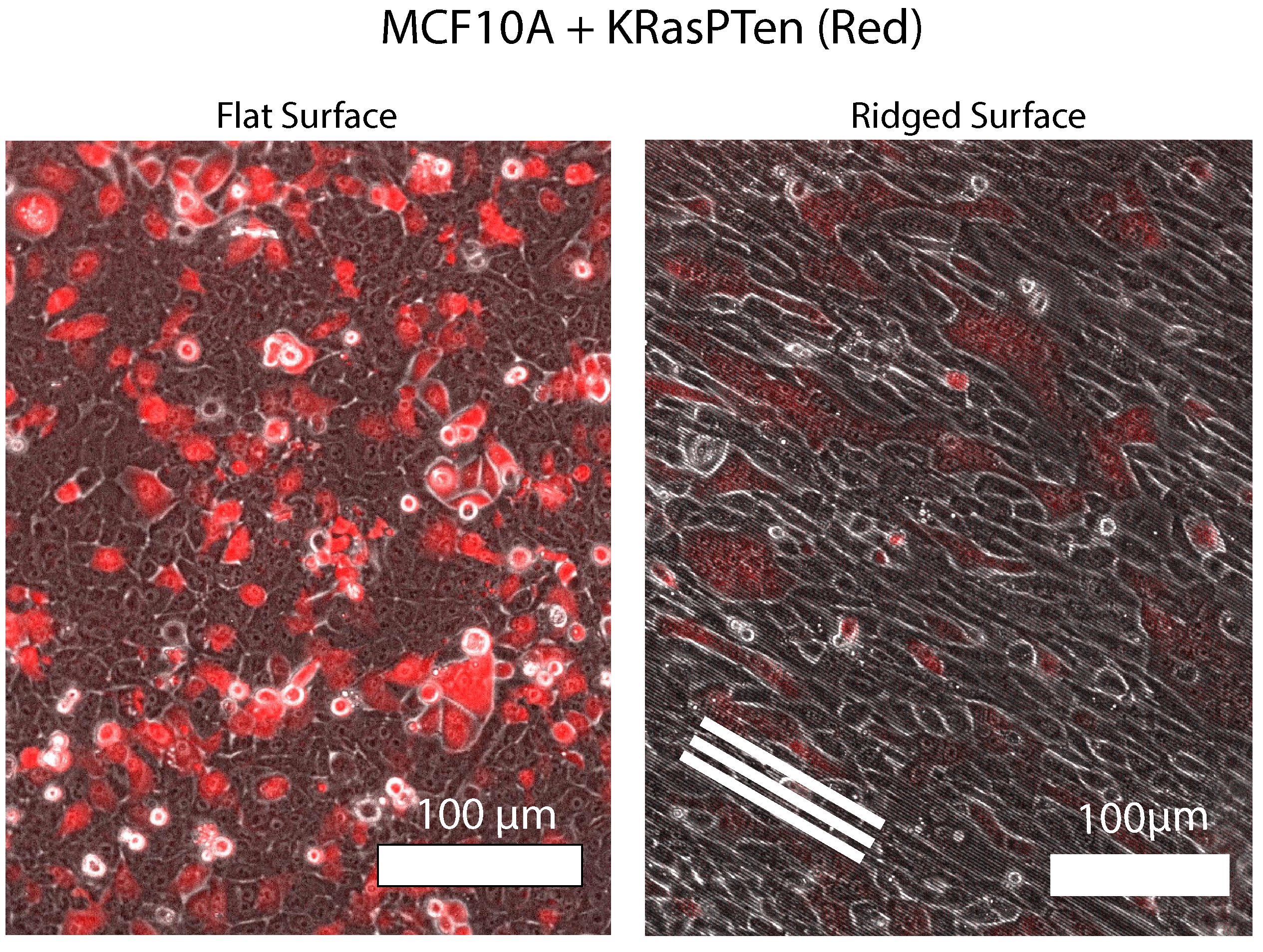
Biophysical forces such as cell-cell adhesion and actomyosin driven cell contractility are well known regulators of cell mixing and clustering within mixtures. However, the effect of surface topology on cell clustering, such as aligned collagen fibers found in tumor micro-environments, is unclear. Using a novel cell tracking pipeline based on the image segmentation results of the AI Cellpose, we are exploring the effect of aligned nanoscale ridges on collective dynamics within mixtures of healthy cells (MCF10A) and highly malignant cells (KRAS-PTEN mutated MCF10A cells).
Using AI-segmented cell tracking, this study investigates how aligned nanoscale ridges, mimicking tumor microenvironment collagen fibers, influence cell mixing and clustering in co-cultures of healthy and malignant cells.
• Rhythmic sharing: A bio-inspired paradigm for zero-shot adaptation and learning in neural networks
Dr. Hoony Kang, Physics
The brain can rapidly adapt to new contexts and learn from limited data, a coveted characteristic that artificial intelligence algorithms have struggled to mimic.
Inspired by oscillatory rhythms of the mechanical structures of neural cells, we developed a learning paradigm that is based on oscillations in link strengths and
associates learning with the coordination of these oscillations. We find that this paradigm yields rapid adaptation and learning in artificial neural networks.
Link oscillations can rapidly change coordination, endowing the network with the ability to sense subtle context changes in an unsupervised manner. In other words,
the network generates the missing contextual tokens required to perform as a generalist AI architecture capable of predicting dynamics in multiple contexts.
Oscillations also allow the network to extrapolate dynamics to never-seen-before contexts. These capabilities make our learning paradigm a powerful starting point for
novel models of learning and cognition. Furthermore, learning through link coordination is agnostic to the specifics of the neural network architecture,
hence our study opens the door for introducing rapid adaptation and learning capabilities into leading AI models.
Publication:
Kang. H, R.M. Lee, W. Losert, arXiv 2025
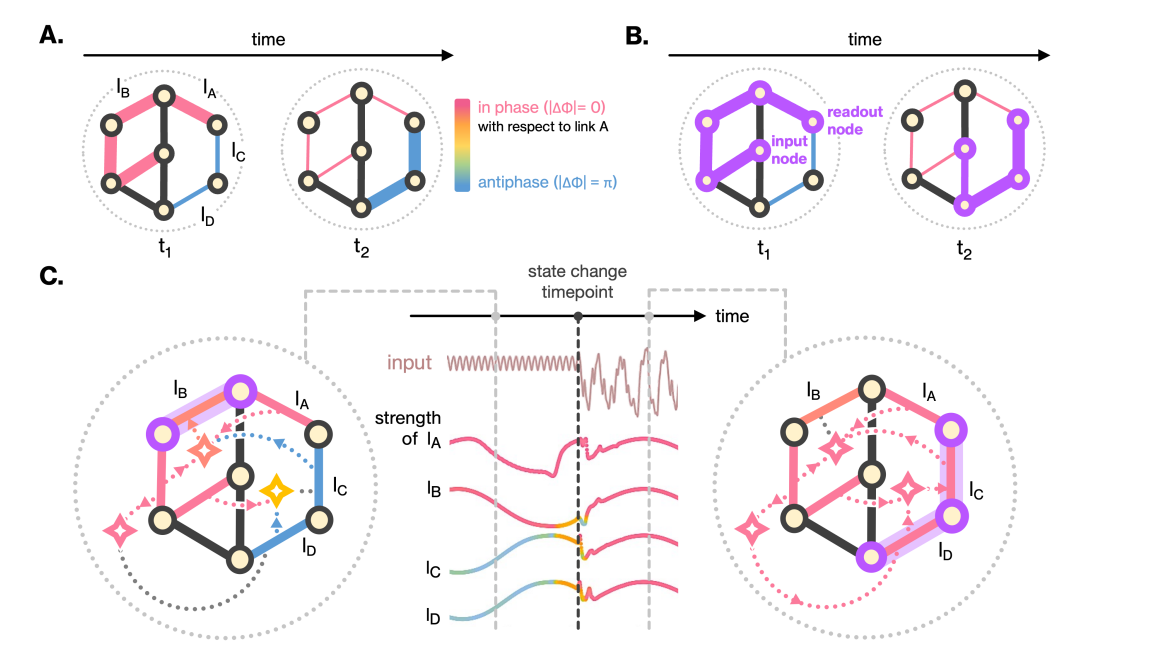
Rhythmic sharing of information via oscillating link strengths. (A) Temporal snapshots of oscillating link strengths.
(B) Information flow rerouted by link oscillations. (C) Input-dependent link activation and synchronization.
• Analysis of Actin Waves using Computer Vision Algorithms
Dr. Leonard Campanello, Physics. Dr. Matthew J. Hourwitz, Chemistry
with Prof. John Fourkas (University of Maryland), and Dr. Rachel Lee, Research Scientist
Quantifying the spatiotemporal dynamics of objects in biological systems often requires the analysis of
diffuse concentration fields, such as fluorescent actin. In this project, we utilize a computer
vision algorithm called “optical flow” to capture and coarse grain the dynamics of amorphous
actin intensity fields to measure physical properties such as velocity and persistence.
We use this analysis to determine the effect of surface topographies on actin dynamics, the effect of
electric fields on neutrophil migration, and the ways that actin waves in Dictyostelium
Discoidium cells change in response to different physical and chemical perturbations.
Publication:
R.M. Lee, L. Campanello, M.J. Hourwitz, P. Alvarez, A. Omidvar, J.T. Fourkas, W. Losert, Molecular Biology of the Cell 2020
HL60 cell with fluorescently labeled actin, overlaid with clustering of optical flow vectors to quantify mesoscale actin dynamics.
• Neurotransmitter regulated macroscale tissue rhythms
Spandan Pathak, PhD Student, Biophysics
with Prof. Jens Herberholz (Department of Psychology, University of Maryland)
To study the regulatory effect of the Central Nervous System (CNS) on intestinal functions, we study motility
patterns in crayfish hind-gut upon neurogenic and chemical excitations. Using Calcium imaging, we also intend
to identify the exact nature of bidirectional communication channels between CNS and ENS (Enteric Nervous
System) and in the process, focus deeper on the gut-brain axis.
Movement of isolated hindgut with nerve N7 attached demonstrates spatio-temporal waves across different scales.
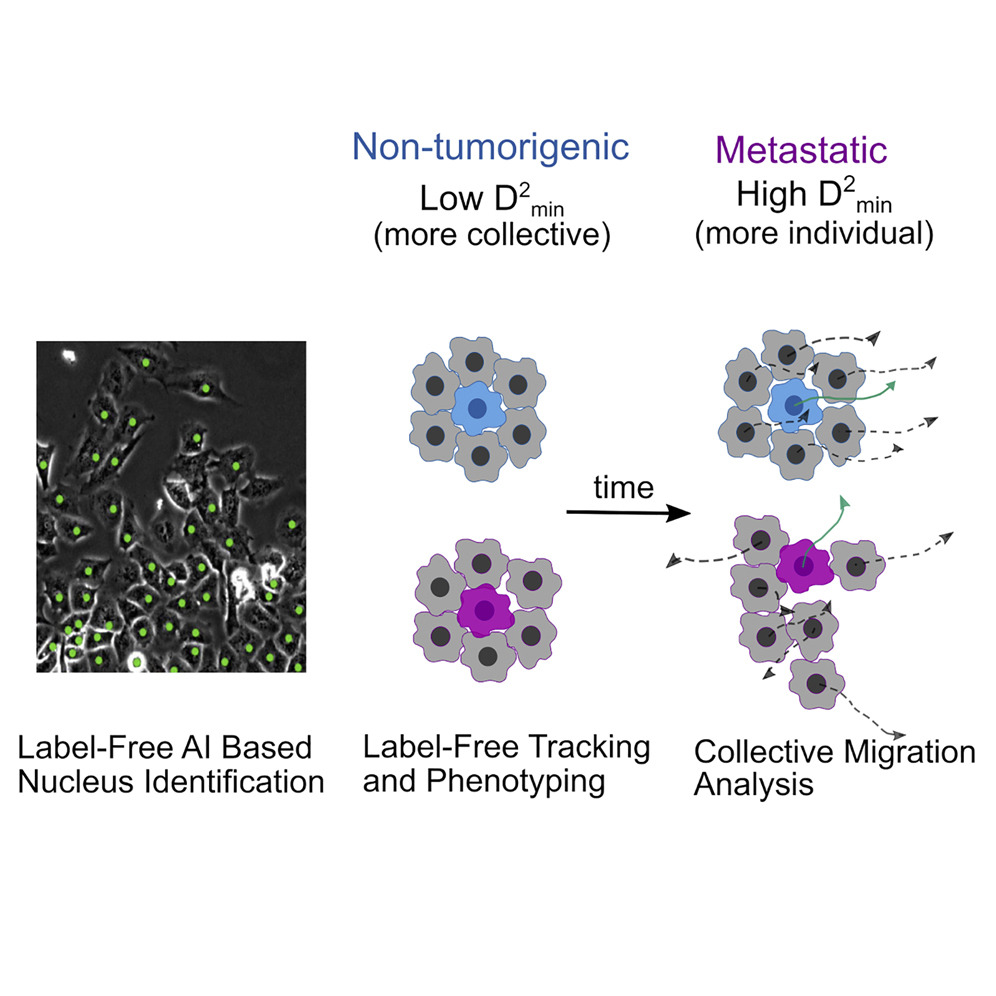
• AI-enabled quantification of Collective Dynamics
Cathy Gu, PhD Student, Physics
with Dr. Joe Chalfoun (NIST), and Prof. Stuart Martin (University of Maryland School of Medicine)
Collective cell migration is an umbrella term for a rich variety of cell behaviors, whose distinct character is important for biological function, notably for cancer metastasis. One essential feature of collective behavior is the motion of cells relative to their immediate neighbors. We introduce an AI-based pipeline to segment and track cell nuclei from phase-contrast images.
Publication:
S. Gu, R.M. Lee, Z. Benson, C. Ling, M.I. Vitolo, S.S. Martin, J. Chalfoun, W. Losert, iScience, iScience 2022
AI-based image analysis pipeline to segment and track cell nuclei from phase-contrast images
• Modeling Shape Dynamics on Nanostructured Surfaces
Corey Herr, PhD Student, Physics
with Dr. John Fourkas (UMD), and Prof. Igor Aronson (University of Maryland School of Medicine)
We are interested in modelling the intercellular dynamics that drive cell motility. In many phase-field models, a deterministic approach is used, however biological systems are extremely noisy. Additionally, many traditional models use simple principles to determine how actin polymerizes and drives the motion of the cell. We used a coupled activator-inhibitor system known as an excitable system that has been experimentally fitted to the dynamics of actin regulatory proteins. We then integrated this stochastic excitable system model into a multi-phase model of cell motility to allow for cell interaction.
Publication:
C. Herr, B. Winkler, F. Ziebert, I.S. Aranson, J.T. Fourkas, W. Losert, Communications Physics 2022
Stochastic multi-phase field model of cell motility using an excitable system for actin dynamics, capturing biological noise and cell interactions.
Maryland Day: Welcome to Cells in Motion!
Lab Outreach
As part of the 2024 Maryland Day event, the Losert Lab created an interactive demonstration of our cell tracking software where participants were able
to track their motion while playing games such as follow the leader. An acoustic leviation device was also demonstrated to trap styrofoam beads in air. To watch our video or to learn more, visit our
Maryland Day page!