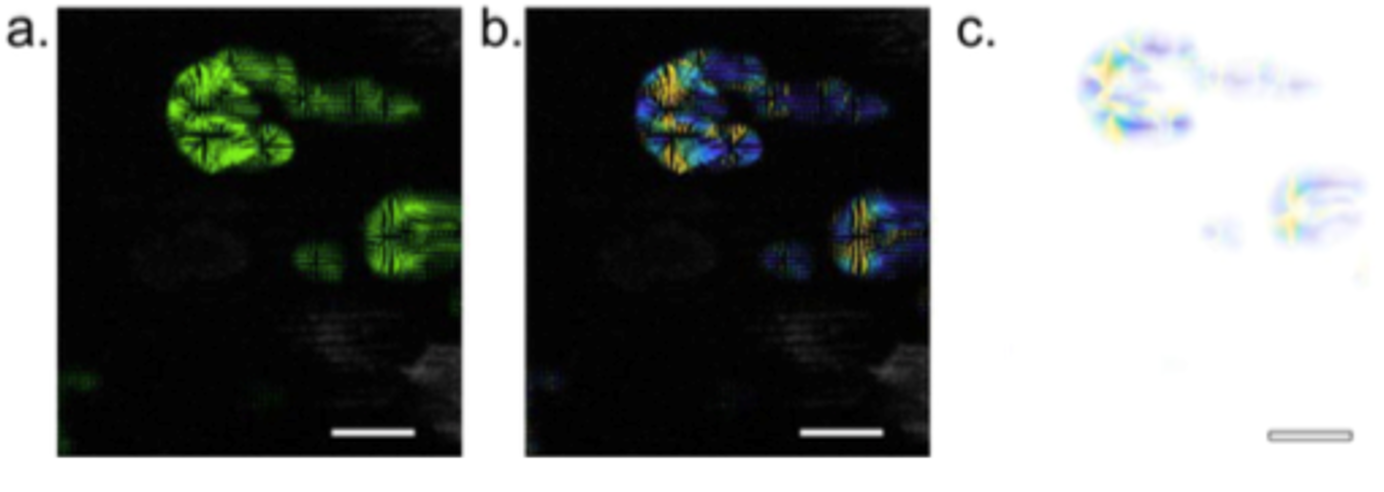
Optical Flow Analysis
We developed an optical flow analysis algorithm to quantify the speed of cytoskeletal and signaling waves obtained by imaging fluorescent biosensors. Our optical flow analysis uses the Lucas-Kanade algorithm to capture the directionality of cytoskeletal and signaling wave dynamics. After clustering the vectors into single objects, Crocker-Grier's Particle Tracking Algorithm can be used to track the wave fronts.